01
2015-08
BrosMed Enters USA Coronary Angioplasty Catheter Market
BrosMed Enters USA Coronary Angioplasty Catheter Market August 1st, 2015, BrosMed announced that BrosMed Medical and US Endovascular Enter into Exclusive Agreement to Distribute Coronary Angioplasty Catheters into USA Market.
2015-08-01
14
2015-04
BrosMed Announces CE Mark and Launch for Minerva™ Balloon Dilatation Catheter
April 14, 2015—BrosMed announced CE Mark and Launch of its Minerva™ percutaneous transluminal angioplasty (PTA) dilatation catheter for the treatment of patients with peripheral arterial disease.
2015-04-14
27
2014-12
On 16th December 2014, Mr. Benny Lee, GM of BrosMed Medical and Mr. Stephen Lee, Deputy GM of BrosMed Medical, held an effective and productive meeting with Mr. Bas van’t Wout, Holland Parliament Member & vice Minister of Health, accompanied by Mr. Jos de Korte, GM of BrosMed B.V..
2014-12-27
24
2014-11
BrosMed Announces the Launch of a New Company Logo and Website
November 24, 2014, we are proud to announce the launch of a new company logo and release our new website, designed with a fresh new look and user-friendly navigation, updated with the latest information about our products and services, as part of the ongoing evolution of our company’s brand and Visual Identity (VI).
2014-11-24
08
2014-10
October 8, 2014— Following the FDA approval of Artimes™ Balloon Dilatation Catheter in September, BrosMed announced FDA 510(k) clearance for Apollo™ Balloon Dilatation Catheter which is now approved and ready for sale in the United States of America and the North America market for the interventional cardiovascular use.
2014-10-08
17
2014-09
September 17, 2014— Following the CE approval and launch Artimes™ Balloon Dilatation Catheter, BrosMed announced FDA 510(k) clearance for Artimes™ Balloon Dilatation Catheter which is now approved and ready for sale in the United States of America and the North America market for the interventional cardiovascular use.
2014-09-17
02
2014-09
BrosMed Establishes Europe Subsidiary in the Netherlands
September 2, 2014, BrosMed Medical has announced the establishment of a subsidiary company in the Netherlands, BrosMed Medical B.V.
2014-09-02
10
2014-07
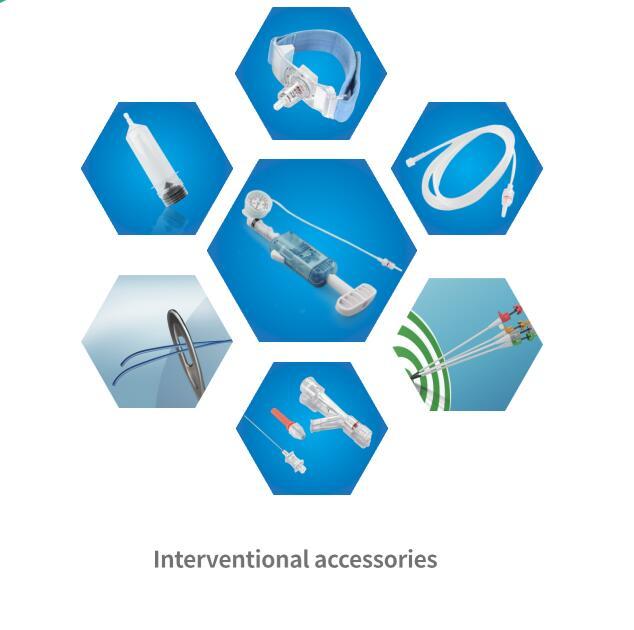
July, 10, 2014— Following the CE approval and launch Artimes™ and Apollo™ Balloon Dilatation Catheter, BrosMed announced CE Mark and Launch of Fourteen (14) types of interventional accessories which are now approved in EU for the cardiology and radiology procedures.
2014-07-10